Soleno Therapeutics Announces Positive Data Showing Continued Significant Improvements in Symptoms of PWS following One Year Treatment with DCCR
Statistically significant reduction in hyperphagia and all other PWS behavioral parameters in Study C602
Statistically significant improvements compared to natural history of PWS from the PATH for PWS Study
On track for data submission to the FDA in Q3 2021
A total of 115 subjects were enrolled into C602, the extension study in patients with PWS who completed DESTINY PWS, an international, multi-center, randomized, double-blind, placebo-controlled study of DCCR in 127 PWS patients at 29 sites in the
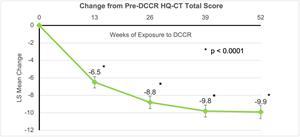
Hyperphagia: The mean (SE) improvement in hyperphagia, the primary endpoint in the DESTINY PWS study, represented by a decrease in the HQ-CT total score, of -9.9 (0.77), was highly significant (p<0.0001) after receiving DCCR for 52 weeks. Highly significant improvements in hyperphagia were also observed after receiving DCCR for 13, 26, and 39 weeks (all p<0.0001).
PWS related behaviors: Behaviors related to PWS were measured using the PWS Profile Questionnaire (PWS-P). After 52 weeks, there were statistically significant improvements in all behavioral domains (all p<0.0001) and after receiving DCCR for 13, 26, and 39 weeks (all p<0.0004).
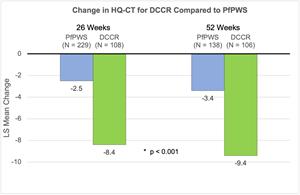
For the comparison of C602 to PfPWS, statistical techniques were employed to minimize and/or reduce selection bias during subject matching and to account for the impact and timing of the COVID-19 pandemic. Matching of subjects to and comparisons with PfPWS were conducted by an external CRO independent of Soleno using a pre-specified Statistical Analysis Plan.
Hyperphagia: PfPWS subjects showed a mean (SE) change of -2.5 (0.38) and -3.4 (0.48) after 26 and 52 weeks compared with that of -8.4 (0.72) and -9.4 (0.74) in matched C602 subjects, showing a statistically significant improvement with DCCR (p<0.001 for both comparisons).
PWS related behaviors: As with hyperphagia, statistically significant improvements with DCCR in C602 subjects compared with matched subjects in the PfPWS study were seen in all behavioral domains of the PWS-P after 26 and 52 weeks (p<0.003 for all).
Additional Results from Study C602 after One Year
Body Composition Changes: Patients in study C602 showed no significant change in body fat mass and statistically significant improvements in lean body mass (p<0.0001) and the ratio of lean body mass to fat mass (p=0.0005).
Endocrine and Metabolic Changes: Subjects demonstrated highly significant improvements in leptin (p<0.0001), adiponectin (p<0.0001), and fasting insulin (p=0.0004). Significant improvement in insulin sensitivity measured using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (p=0.03) was also observed.
Safety: The safety profile of DCCR remains consistent with the known safety profile of diazoxide and the prior experience with DCCR with increases in blood glucose levels, hypertrichosis and peripheral edema being the most common adverse events. No serious, unexpected, related adverse events have occurred with DCCR in the program to date.
“Hyperphagia is one of the main problems associated with PWS and can have life-threatening consequences such as severe obesity. No treatments are currently available,” said Dr.
“The PATH for PWS study is an ongoing evaluation of the natural history of individuals with PWS,” said Dr.
“We are extremely pleased with these results from our ongoing open-label extension study and their comparison to the PATH for PWS study,” said Anish Bhatnagar, M.D., Chief Executive Officer of Soleno Therapeutics. “We look forward to submitting the data from these studies to the
Soleno will submit these data to the FDA as part of an ongoing discussion with the Agency regarding the clinical data necessary to support the submission of a New Drug Application (NDA) to market DCCR for the treatment of PWS. The FDA has previously conveyed to Soleno that another clinical trial will likely be needed and that open-label data and comparisons with natural history sources such as PATH for PWS may have statistical and other limitations, but it has agreed to review the data to determine whether it is appropriate for the Company to submit an NDA.
For further information about DESTINY PWS (NCT03440814) and the open-label extension study Study C602 (NCT03714373), please visit: www.clinicaltrials.gov.
About PWS
The Prader-Willi Syndrome Association USA estimates that PWS occurs in one in every 15,000 live births in the U.S. The hallmark symptom of this disorder is hyperphagia, a chronic feeling of insatiable hunger that severely diminishes the quality of life for PWS patients and their families. Additional characteristics of PWS include behavioral problems, cognitive disabilities, low muscle tone, short stature (when not treated with growth hormone), the accumulation of excess body fat, developmental delays, and incomplete sexual development. Hyperphagia can lead to significant morbidities (e.g., obesity, diabetes, cardiovascular disease) and mortality (e.g., stomach rupture, choking, accidental death due to food seeking behavior). In a global survey conducted by the Foundation for Prader-Willi Research, 96.5% of respondents (parent and caregivers) rated hyperphagia and 92.9 % body composition as the most important or a very important symptom to be relieved by a new medicine. There are currently no approved therapies to treat the hyperphagia/appetite, metabolic, cognitive function, or behavioral aspects of the disorder.
About DCCR (Diazoxide Choline) Extended-Release Tablets
DCCR is a novel, proprietary extended-release dosage form containing the crystalline salt of diazoxide and is administered once-daily. The parent molecule, diazoxide, has been used for decades in thousands of patients in a few rare diseases in neonates, infants, children and adults, but has not been approved for use in PWS. Soleno conceived of and established extensive patent protection on the therapeutic use of diazoxide and DCCR in patients with PWS. The DCCR development program is supported by data from five completed Phase 1 clinical studies in healthy volunteers and three completed Phase 2 clinical studies, one of which was in PWS patients. In the PWS Phase 3 study, DCCR showed promise in addressing hyperphagia, the hallmark symptom of PWS, as well as several other symptoms such as aggressive/destructive behaviors, fat mass and other metabolic parameters. Diazoxide choline has orphan designation in the
About Soleno Therapeutics, Inc.
Soleno is focused on the development and commercialization of novel therapeutics for the treatment of rare diseases. The company’s lead candidate, DCCR extended-release tablets, a once-daily oral tablet for the treatment of Prader-Willi Syndrome (PWS), is currently being evaluated in a Phase 3 clinical development program. For more information, please visit www.soleno.life.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical facts contained in this press release are forward-looking statements, including statements regarding the adequacy of our clinical data to support a marketing application and/or approval of such an application, the timing and likelihood of acceptance of such an application, the timing and likelihood of approval of the application, and potential interactions with the FDA regarding the determination of a path forward for DCCR for the treatment of PWS. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "contemplates," "believes," "estimates," "predicts," "potential" or "continue" or the negative of these terms or other similar expressions. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions, including the risks and uncertainties associated with market conditions, as well as risks and uncertainties inherent in Soleno’s business, including those described in the company's prior press releases and in the periodic reports it files with the SEC. The events and circumstances reflected in the company's forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, the company does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Corporate Contact:
212-915-2578
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/25bb5fe4-42a4-4598-a799-2cd46967e357
https://www.globenewswire.com/NewsRoom/AttachmentNg/8b602755-a0f9-4c9e-92f2-252a66397e6e

Source: Soleno Therapeutics